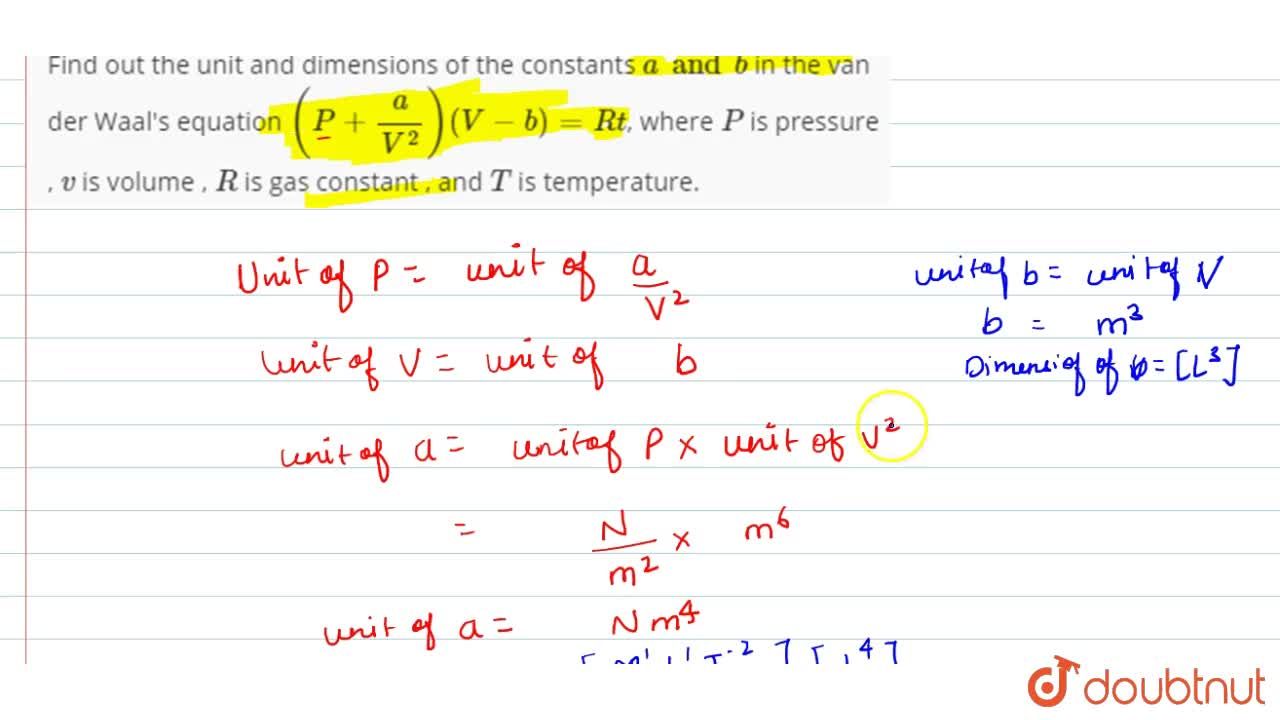
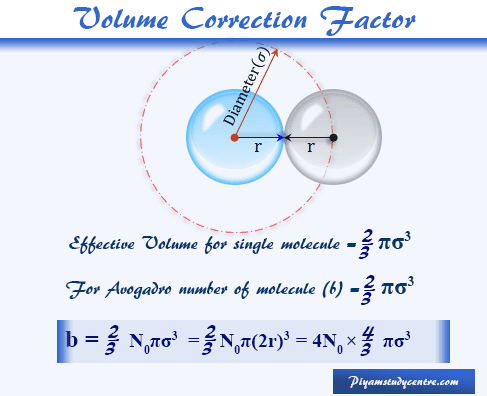
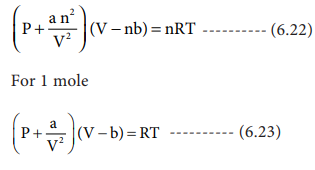The van der waals equation for a gas is (P + a/v2)(V-b) = RT where P = Pressure, V = - Physics - Units And Measurements - 2236057 | Meritnation.com
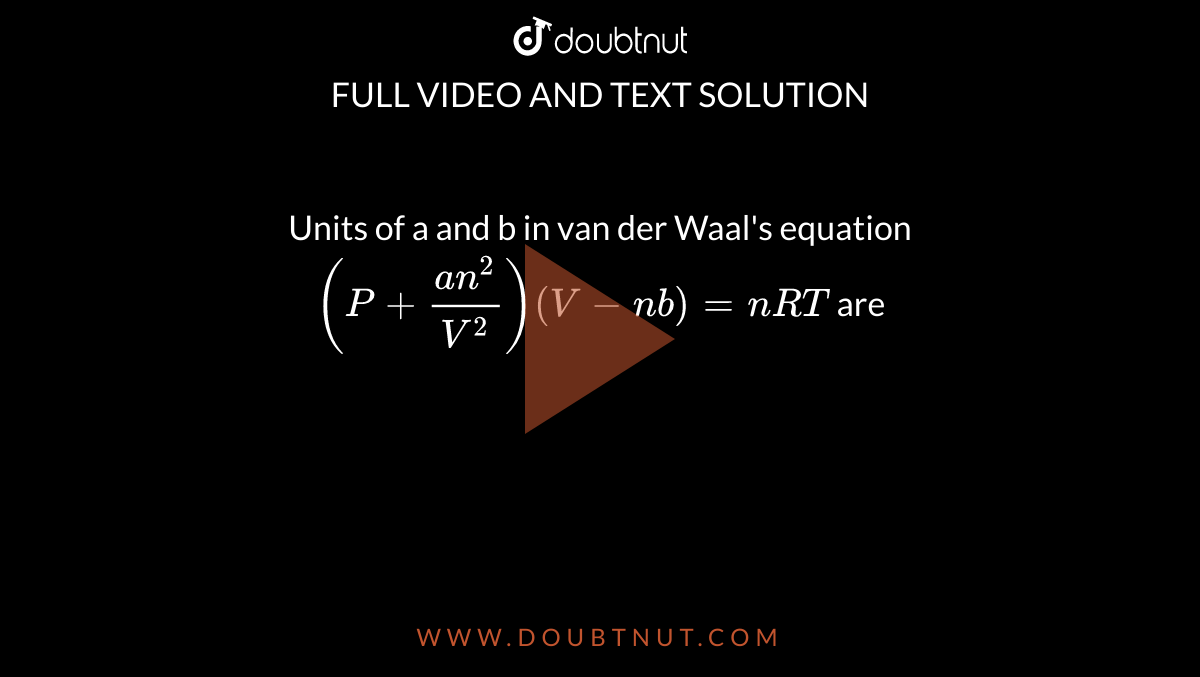
What is the unit of 'a' in terms of fundamental units in Van der waal's equation (P+(a)/(V^(2)))(V-b)=RT?
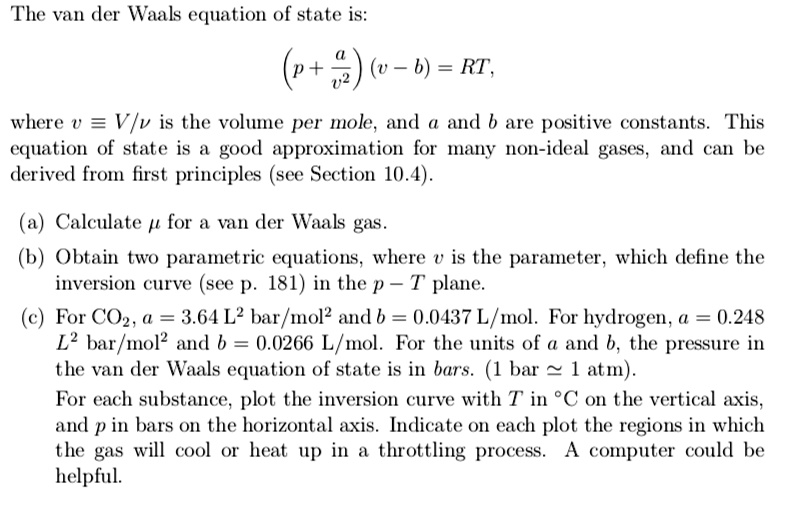
SOLVED: The van der Waals equation of state is: (0- 6) = RT, where v = Vlv is the volume per mole, and and 6 are positive constants. This equation of state