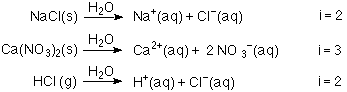At what temperature would 1.3 m NaCl freeze, given that the van 't Hoff factor for NaCl is 1.9? Kf for water is 1.86 degrees C/m. | Homework.Study.com

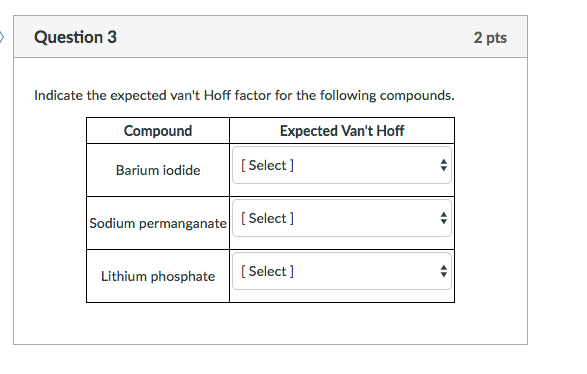
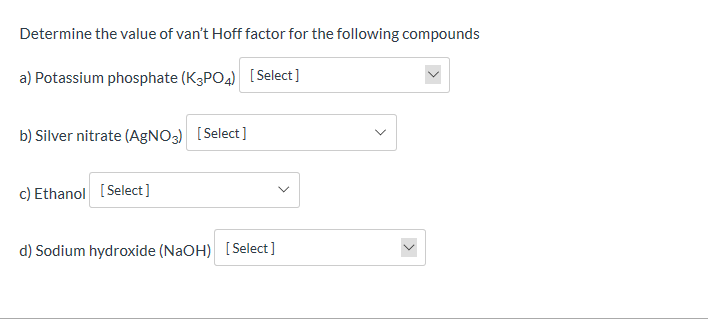
OneClass: Indicate the expected van't Hoff factor for the following compounds. Sodium carbonate Cesiu...

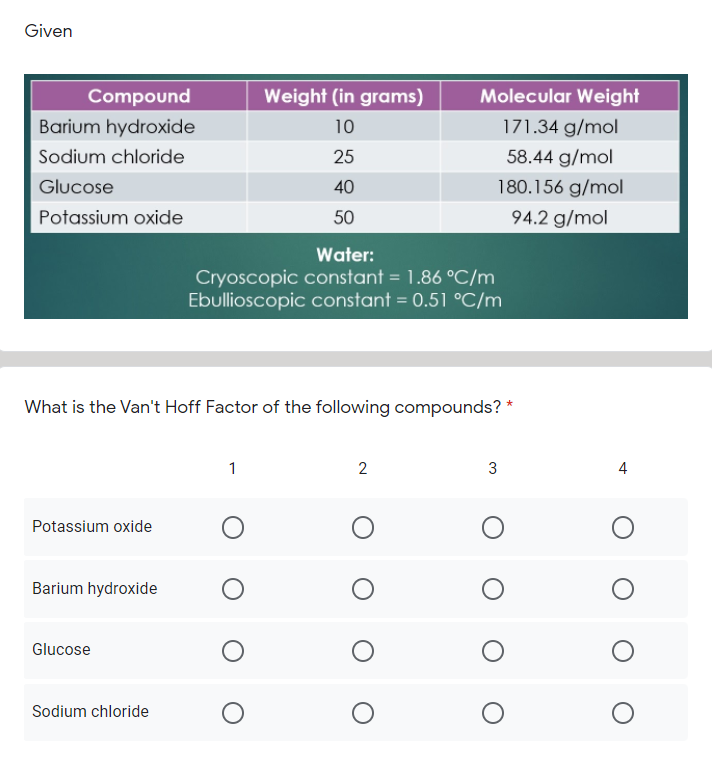
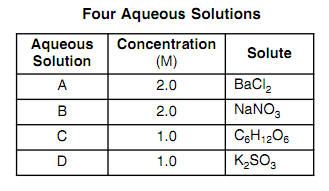
The following data were collected for three compounds in aqueous solution. Determine the value of the van't Hoff factor for each salt (Kf for water = 1.86 oC/m) for the experimentally measured ?
What is the van 't Hoff factor for the NaCl in aqueous solution (assuming the complete dissociation of NaCl)? - Quora

SOLVED:The van't Hoff factor for KCl is i=1.85 . What is the boiling point of a 0.75 m solution of KCl in water? For water, Kb=0.51(^∘ C ·kg) / mol

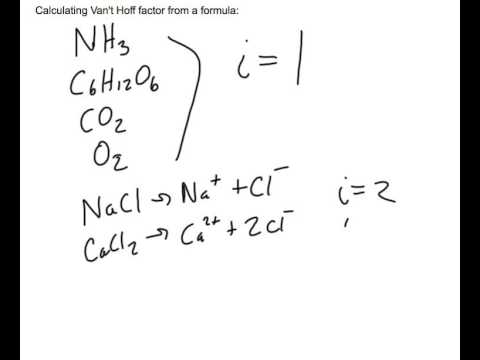
Sooo how the hell am I supposed to calculate the van't hoff factor? Isn't it just 2... for every single one? What is there to “calculate”? Please help, the lab notes say

Van't Hoff factor as a function of concentration (calculated according... | Download Scientific Diagram